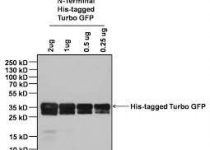
Cusabio N-terminal 10xHis-B2M-tagged Recombinant
Species: Homo sapiens (human)
Fountain: E. coli
Label information: N-terminal 10xHis-B2M-tagged
Estimated response time: 7-11 business days
Protein type: recombinant protein
Gene name: LY6G6D
Alternative names
Megakaryocyte-enhanced gene transcript 1 protein; C6orf23, G6D, MEGT1, NG25
Uniprot: O95868
Expression Region: 20-104yy
Sequence information: Total length of the mature protein
Theoretical MW: 25.5kDa
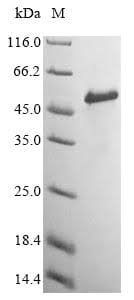
Purity
More than 85% as determined by SDS-PAGE.
Storage buffer
If the dosage form is liquid, the default storage buffer is a Tris/PBS based buffer, 5%-50% glycerol. If the administration form is a lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer, 6% trehalose, pH 8.0.
Endotoxin Level: Not tested
Shipping condition: ice packs
Storage
Short term: -20°C; Long life: -80°C. Minimize freeze-thaw cycles.
Date of Expiry: 1 year
Investigation area: Others
Restriction
For research use only. Not for use in diagnostic procedures, drug use, or for administration to humans or animals.
Abstract
Human N-terminal 10xHis beta2 microglobulin (B2M)-tagged Recombinant is the light chain of the class I molecule of the major histocompatibility complex (MHC). High-yield production of this protein is a prerequisite for MHC I tetramer preparation. The present study aims to obtain recombinant human beta2m expressed in Escherichia coli (E. coli), in order to prepare class MHC tetramers. I. For the cloning of the human beta2m gene, a specific primer pair was designed based on the published sequence of this gene and the cDNA of the complete coding region of the precursor beta2m was obtained by RT-PCR from the total RNA of human leukocytes.
The amplified cDNA was subsequently cloned and its sequence was confirmed by DNA sequencing analysis (sequence has been deposited in GenBank under accession number AY187687). The prokaryotic expression vector containing a gene encoding mature beta2m was constructed by inserting the DNA fragment, which was generated by PCR reaction with the cloned beta2m gene as a template, into an IPTG-inducible expression vector plasmid pET-3c. The first eight codons for the N-terminal amino acid residues of beta2m were optimized for expression in E. coli. The complete sequence of the beta2m gene in the expression vector was verified by DNA sequencing analysis.
High-yield expression of beta2m was achieved in E. coli transformed with the expression vector, and most of the recombinant beta2m existed in the inclusion body after induction with IPTG. The inclusion body was washed extensively and beta2m in the inclusion body was solubilized with 8 mol/L urea. Beta2m was refolded by dialysis and purified by ion-exchange chromatography (Q-Sepharose). Western blot assay indicated that the human native beta2m polyclonal antibody could specifically react with the recombinant protein.
The purified protein appeared as a single band on both SDS-PAGE and Western Blot, indicating that it was chemically and antigenically pure. This work establishes a convenient approach for the renaturation and purification of a large amount of recombinant beta2m that is identical to the native protein with no fused tags except for a methionine residue at the amino terminus. This provides the basis for the preparation of MHC tetramers.